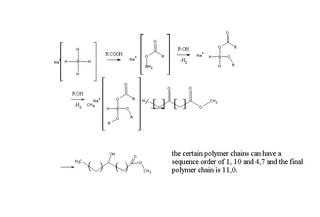
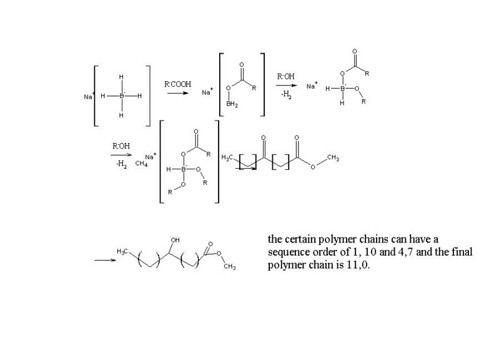
Methyl 3-, 6- and 13-oxo tetradecanoates went through reduction with NaBH4 in the presence of 1,2:5,6-di-O-isopropylidene-Dglucofuranose (DIPGH) and menthol together with isovaleric and pivalic acids in THF solution. This work signifies the importance of positional
effect. The position of lower steric hinderance and higher enantiomeric excess and asymmetric reduction yield were noted down, namely the prochiral 13-keto isomer structures.
With this asymmetric reduction at normal atmospheric pressure together with inexpensive auxiliaries make it competitive with other reduction methods and is needed to assess the need in the market. Here's a link for this article
Wednesday, August 6, 2008
Asymmetric synthesis of monohydroxy tetradecanoic acids chymistry
Labels:
chemistry info
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment