
This is en example of what we learned in class, the Markovnikov hydration of n-propyne (a terminal Alkyne) that produces a Ketone. Using ACS publications, i found an article that describes an ideal anti-Markovnikov hydration of the same compound, to produce an Aldehyde instead.
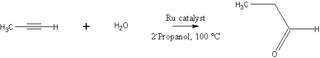
To induce this reaction, the catalyst cyclopentadienylruthenium is used in a 2-Propanol solvent at 100 deg C, with yields over 99% for this reaction, other Alkynes bearing over 90% for the most part.
full article
No comments:
Post a Comment