Hi Dr Bradley,
I've already fixed my post and uploaded the images.
I did not delete any comments that you made. On Wednesday after I posted the assignment, I checked the blog many times on the day and made some changes. But I still saw "No comments". I actually never delete any comments.
Do I need to correct anything else from my assignment? Do I need to redo it?
Thank you!
Tuesday, August 12, 2008
Question about blog assignment chemistry
The Role of Phosphate Esters in Biochemistry (DNA and Cellular Processes) chemistry
The backbone of deoxyribonucleic acid (DNA) is composed of nucleic acids, which are made up of ribofuranoside units strung together by phosphate esters. These phosphodiester bonds (two ester bonds) create the DNA backbone.
This simple ChemSketch shows the linking of nucleotides in DNA via phosphate ester groups:
Phosphate esters are created when phosphoric acid and an alcohol combine. Here is an example with methanol (alcohol), which can form three esters based on how many moles of methanol are used:
In addition to its role in the backbone of DNA, phosphate groups play a biochemical role in ribosome-substrate interactions and the regulation of cellular processes. The regulation of their formation on key body proteins regulates these processes, and malfunctions in their regulation can result in cancer, diabetes, and even obesity.
Phosphate groups also play a major part in the bending of the DNA backbone, due to the repulsion of the negative charges. Other experimentation concerning the role of phosphate ester groups includes looking at their electrostatic contribution to the free energy of the bent DNA backbone as well as the synthesis of heparin-immobilized polyetherurethanes, whose side groups have hydrolysable ester groups (heparin being a synthetic anti-coagulent).
Here is reference one and reference two and reference three from CiteULink.
In addition, Chapter 11 and Chapter 23 from Wade provide substantial information on this topic.
anti-Markovnikov hydration of an Alkyne chemistry

This is en example of what we learned in class, the Markovnikov hydration of n-propyne (a terminal Alkyne) that produces a Ketone. Using ACS publications, i found an article that describes an ideal anti-Markovnikov hydration of the same compound, to produce an Aldehyde instead.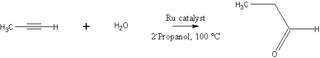
To induce this reaction, the catalyst cyclopentadienylruthenium is used in a 2-Propanol solvent at 100 deg C, with yields over 99% for this reaction, other Alkynes bearing over 90% for the most part.
full article