Along with the growing interest in Fuel Cell powered cars comes the need for higher production methods of Hydrogen, both in bulk form and in-car conversions (for fuels such as methanol and ethanol to be converted to hydrogen on board). Previous methods of converting Ethanol to hydrogen was by means of high-temperature steam reformation (at temperatures in excess of 600° C) to produce Hydrogen gas and CO.
This journal describes a special method of low-temperature dehydrogenation of ethanol over special Raney catalyst with Cu added to it. The first step produces one mole each of hydrogen gas and acetaldehyde (per mole of ethanol). This is followed by the decarbonylation of acetaldehyde to form methanol and CO. The whole reaction undergoes a water-gas shift to net one mole each of Methane and Carbon dioxide and two moles of Hydrogen.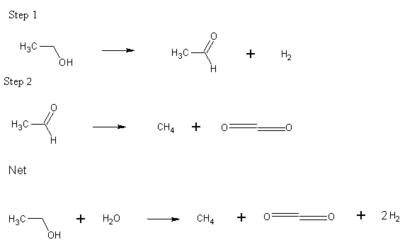
Compared to high temperature reformation methods, which produces 6 moles of hydrogen per mole of ethanol, this reaction doesn't seem as fuel efficient, though the authors were confident, that with an internal combustion engine on-board that uses the methane produced as fuel, the total output energy would be equal.
Citeulike Link
Wednesday, August 6, 2008
Production of Hydrogen by means of hydrogenation of ethanol chymitry
Labels:
chemistry info
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment