Almost everyone has seen a lightstick. A lightstick is a plastic tube with a glass vile inside it. When the tube is bent, the vial breaks allowing the chemicals to mix and react. The colorfully glowing sticks utilize a chemical process called chemiluminescence where energy is released in the form of light. The most common lightsticks use chemiluminescence with colored tubes to provide the desired color.
This process is not caused by heat and may not produce heat, but the speed of reaction is still dependence on environmental heat. The colder the environment, the slower the reaction and will glow longer.
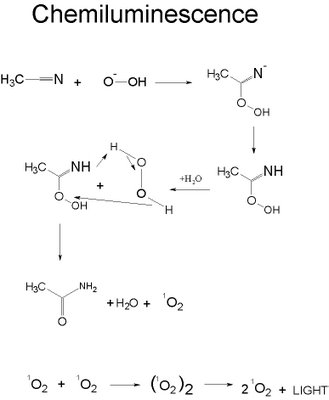
Lightsticks have three parts. There are two chemicals that react to release energy which is converted to light. Usually, commercial lightsticks utilize the reaction between hydrogen peroxide and acetonitrile. When the glass vile is broken and the two chemicals are mixed, it will release enough energy to excite the electrons in the oxygen to cause the electrons to jump to a higher energy level and then fall back releasing light.
Specifically, the hydrogen peroxide oxidizes the acetonitril eventually forming excited oxygen. This decomposes and releases the energy as light as can be seen stepwise above.
More on chemiluminescence can be found here on “A Chemiluminescence Reaction between Hydrogen Peroxide and Acetonitrile and Its Applications.”
Wednesday, August 6, 2008
The Chemistry Behind Lightsticks chemistry
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment