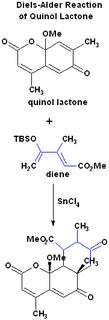
This is a Diels-Alder reaction, which does not follow the regiospecificity rules. What makes this reaction unusual is that normally quinol-lactone does not react with dienes. Also if we follow the regiospecificity rules we would logically expect to get the structure in Figure 2. However by using stannic chloride (SnCl4) in methylene chloride, the product shown was obtained. It was determined through NMR spectroscopy that this particular product was present, and not its isomer in Figure 2. TBSO stands for the tert-butyldimethylsilyl group.
The reaction is described in this article.
I found an article which looks more closely at the process of migration and elimination of TBS when the final product is synthesized, which produces TBSOTf. It states that stannic chloride causes the migration of the tosyl group (1st paragraph of "Results and Discussion" section). I believe this plays a role in the final "inversion" of the product in the posted reaction. Also this Diels-Alder reaction is performed in Lewis acid which gives the regioselectivity opposite to what we would expect in an uncatalyzed reaction.
The product in Figure 2 should be expected, because if we examine the ortho-para rules for regioselectivity through the formation of radicals without a catalysit (Lewis acid), we can see that the major product should resemble the one in Figure 2. There is an example on this page under the "Regioselectivity" section, which has the major product boxed in.
Wednesday, August 6, 2008
Diels-Alder reaction of Quinol Lactone chymistry
Labels:
chemistry info
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment