Hi Dr Bradley,
I've already fixed my post and uploaded the images.
I did not delete any comments that you made. On Wednesday after I posted the assignment, I checked the blog many times on the day and made some changes. But I still saw "No comments". I actually never delete any comments.
Do I need to correct anything else from my assignment? Do I need to redo it?
Thank you!
Tuesday, August 12, 2008
Question about blog assignment chemistry
The Role of Phosphate Esters in Biochemistry (DNA and Cellular Processes) chemistry
The backbone of deoxyribonucleic acid (DNA) is composed of nucleic acids, which are made up of ribofuranoside units strung together by phosphate esters. These phosphodiester bonds (two ester bonds) create the DNA backbone.
This simple ChemSketch shows the linking of nucleotides in DNA via phosphate ester groups:
Phosphate esters are created when phosphoric acid and an alcohol combine. Here is an example with methanol (alcohol), which can form three esters based on how many moles of methanol are used:
In addition to its role in the backbone of DNA, phosphate groups play a biochemical role in ribosome-substrate interactions and the regulation of cellular processes. The regulation of their formation on key body proteins regulates these processes, and malfunctions in their regulation can result in cancer, diabetes, and even obesity.
Phosphate groups also play a major part in the bending of the DNA backbone, due to the repulsion of the negative charges. Other experimentation concerning the role of phosphate ester groups includes looking at their electrostatic contribution to the free energy of the bent DNA backbone as well as the synthesis of heparin-immobilized polyetherurethanes, whose side groups have hydrolysable ester groups (heparin being a synthetic anti-coagulent).
Here is reference one and reference two and reference three from CiteULink.
In addition, Chapter 11 and Chapter 23 from Wade provide substantial information on this topic.
anti-Markovnikov hydration of an Alkyne chemistry

This is en example of what we learned in class, the Markovnikov hydration of n-propyne (a terminal Alkyne) that produces a Ketone. Using ACS publications, i found an article that describes an ideal anti-Markovnikov hydration of the same compound, to produce an Aldehyde instead.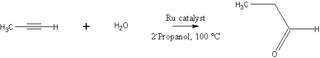
To induce this reaction, the catalyst cyclopentadienylruthenium is used in a 2-Propanol solvent at 100 deg C, with yields over 99% for this reaction, other Alkynes bearing over 90% for the most part.
full article
Nitroglycerine synthesis chemistry
This is a nitrate ester made by esterification. In this process, glycerols reacts with Nitric Acid under a catalyst of Sulphuric Acid at 300oC to yield an ester complex of glyceryl trinitrate otherwise known as nitroglycerine, and three water molecules. In this process, there has been formation of an ester bond (COO). This production is very crucial for medical purposes. Nitroglycerine is used to relieve angina, a condition when the heart isn’t receiving much oxygen. Also, Nitroglycerine was used in a study with yohimbine/l-arginine combination to see the treatment of erectile dysfunction in males. The metrics used in this study were the male’s systolic and diastolic blood pressures. This study created a correlation between the effects of erectile dysfunction with the inadvertent cause of coronary artery disease. Intravenous Nitroglycerine was used to help in the study with the chemical compositions with arginine. Here is a reference for this article on its uses.
Tutoring chemistry
The Drexel Learning Center is offering tutoring for organic chemistry students this term. If you find this to be useful share your experience here.
Synthesis of Ethylene Oxide as a fumigant chemistry
This shows the synthesis of ethlyene oxide which can be used as a fumigant for foods, textiles, soils and for sterilizing biomedical instruments. It readily diffuses through materials without damanging them. Its antibacterial effect is probably due to its ability to alkylate critical cellular enzymes.
Heres a link
repeating mistakes chemistry
Before you post take a look at the comments I made on all of the other students' posts. I see the same errors coming up in the last few posts. In order to get full marks you must:
1) make sure your molecules and reagents are big enough to view easily from http://chem242.blogspot.com. See the first few examples that I graded "full marks" to see how big is big enough
2) you must use CiteULike
3) ALL of the reference info needs to be present in CiteULike (authors, journal name, year, page, volume, title, abstract)
4) the single step reaction that you have in your scheme MUST be in the article you are citing
I will shortly stop giving this feedback repeatedly and just grade it accordingly at each deadline.
If any student has tips (especially for making molecules the proper size in Chemsketch) it would be great if you could record a 1-2 minute screencast of it. Just download the trial version of Camtasia, save as an avi and I'll give you instructions to upload.
Friday, August 8, 2008
Blog Picture Suggestions chemistry
Two things that I did to help add clarity to my images was:
1) In Flikr, before you obtain the URL click on the image which is bigger than the one that shows up in your album and then go to properties to obtain the URL. This image is larger to when Blogger formats it, the picture is more clear.
2) To make the picture even more clear: go into "Edit HTML" of your blog post when you edit it. Then find the line for your image that will look like this:
"http://photos18.flickr.com/22901961_b9fc681f83.jpg?v=0">="DISPLAY: block; MARGIN: 0px auto 10px; WIDTH: 320px; CURSOR: hand; TEXT-ALIGN: center" alt="" src="http://photos18.flickr.com/22901961_b9fc681f83.jpg?v=0" border="0" /
and delete the text in pink (width) from the HTML code. This will make the image a little more clear. If your image is too large though, it will extend out into the navigation on the right so make sure when you delete width that your image doesn't do that.
Addition of Grignard Reagents to Ketones chemistry
------------------------
This is an example of the reaction of a ketone with a Grignard reagent, which gives a tertiary alcohol. The paper presents a study of several different idol-3-ones reacting with Grignard reagents. Idol-3-ones are potentially useful intermediates in the synthesis of alkaloids and pharmaceutical agents.
Going one step further: due to the lack of stability in the tertiary alcohol, a rearrangement is observed on the alcohol molecule, creating a gain in resonance stabilization in the final molecule. The study examined a variety of conditions under which the rearrangement occurred, in order to recognize the most efficient one. It was determined that the rearrangement took place with great facility under acidic or basic conditions or was thermally induced.
full text
or
CiteULike Quick Reference
The dioxygenase-catalysed formation of vicinal cis-diols chemistry
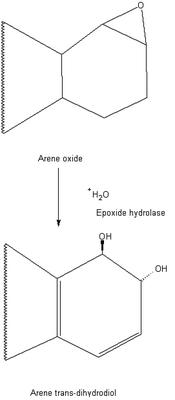
This is an example of the reaction of an epoxide ring reacting with water and and enzyme (epoxide hydrolase) to create a vicinal diol. The paper displays experiments with several different compounds and the role of mono- and di-oxygenase enzymes in arene
metabolism. The paper also displays a variety of intermediary or alternative pathways the the substrates can take to generate different, but useful products. The primary focus is on the impressive ability of bacterial oxygenases to catalyse the cisdihydroxylation
of a diverse range of arenes and alkenes to yield a single enantiomer.
Full Text
or
CiteULink Reference
First deadline grades chemistry
The first deadline for the blog assignment has now past. Everyone who posted has been graded. There is nothing you can now do to improve your grade on the first assignment. Learn from your mistakes and the mistakes of other students by reading my comments on all the posts to make the next deadline in 2 weeks.
One general comment: use a specific reaction of a specific compound that you find in the article. Avoid drawing general structure with R groups.
Tests chemistry
Dr. Bradley,
When are the dates for the test 1 and 2 and the final test date?
Avik
Wednesday, August 6, 2008
Creating Amphetamines by Tosylation of Alcohols chymistry
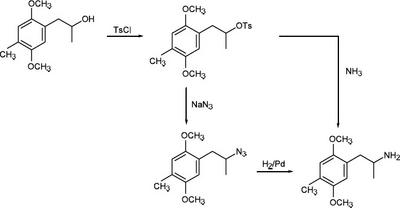
The picture above shows a few of the steps in the creation of amphetamines. In these steps, tosyl chloride is added to (2,5-dimethoxyl-4-methylphenyl)-2-propanol to create the tosylate. After this step, the reaction can proceed in one of two ways. If the chirality of the amphetamine is not important, ammonia is added to the tosylate to give 2,5-dimethoxy-4-methylamphetamine. This reaction has an 80% yield, but has a racemic mixture of products because it is thought to be an SN1 reaction. If the chirality is important, the tosylate is converted into an azide with sodium azide, then hydrogenated using a paladium catalyst to form 2,5-dimethoxy-4-methylamphetamine. Forming the amphetamine using this method gives a final yield of about 77%. The chirality of the original alcohol is inverted by the tosylation, so reacting an (S)-alcohol with the tosyl-azide-hydrogen sequence would give an (R)-amphetamine, and vice versa.
For the full text of the article describing this process, see this report.
Chap 9 UT map ready for testing chymistry
We have quiz 9 in Unreal Tournament 2004 map format available for testing. If you have UT2004 just download the map and textures here.
I'll make these maps available on the network shortly but for now you have to download and play on your computer.
You can play with or without bots (or other players). So if you just want to calmly explore select no bots and don't play on the network.
If you don't have UT2004 but still want to play let me know and we can make arrangements.
Please report any errors or problems as comments on the Edufrag blog on the post of the map you are playing.
This is an example of a student assignment done as a map instead of a blog post.a
A simple one-pot synthesis of β-alkoxy alcohols from alkenes chymistry
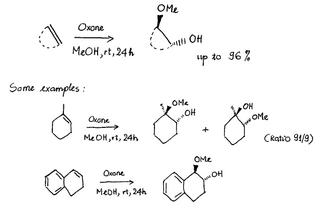
The main protocol for the synthesis of β-alkoxy alcohols is the alcoholysis of 1,2-epoxides. To synthesize epoxides, we can use oxone in the presence of transition metal complexes, or cyclodextrines, or via the formation of dioxiranes.
An application of this type of reaction is the synthesis of β-methoxy alcohols. It is done by the one-pot reaction of alkenes with oxone in methanol without any other catalyst.
Note: Oxone (2KHSO5·KHSO4·K2SO4) is the registered trademark from Du Pont.
General reaction and some examples are shown above.
For reference, please click on http://www.citeulike.org/user/hongan1985/article/258103
Methanol Synthesis and Dehydration Combined in a Single-Stage DME Synthesis Process chymistry
Dimethyl ether (DME) is a multipurpose clean fuel and chemical feedstock that can be produced from a wide variety of of sources and has a number of important applications. About 10,000 tons of DME are manufactured each year for uses in cosmetics and aerosal paint propellants. Its new use as a clean fuel source is gaining attention and research, as it contains no sulfur or nitrogen compounds, has a very low toxicity, and is not corrosive to metals. It can be stored and transported as a liquid at low temperatures
A single-stage, liquid phase synthesis process for DME in a slurry phase reactor system is efficient and facilatates heat removal. The combination of methanol synthesis and methanol dehydration reversible reactions in a single step is thermodynamically more favorable. The liquid phase operation allows for better heat management and higher yields of DME.
The first pictured reaction shows the methanol synthesized from carbon dioxide and it is combined with the second pictured reaction into the last pictured reaction, in which the synthesized methanol is dehydrated to produce DME.
See here for the CiteULike with the reactions and here for another journal article about DME. In addition, the catalytic synthesis of methanol is covered in Ch.10 of Wade and DME itself is discussed in Ch.14
Lucas Reagant chymsitry
The reaction seen above is an example of a substitution nucleophilic 1 reaction. The mild reaction conditions and high yields makes the Lucas reaction a convient way to be utilized in the industrial-scale preparation of alkyl chlorides from aliphatic alcohols. However, the wide use of the Lucas reagent in industrial settings is limited due to the cost of ZnCl2. This reaction proceeds rapidly and is needed for the fast demand and industrial use of menthyl chloride.
Williamson Synthesis of Ethers chymistry
The above reaction is an example of a Williamson synthesis of an ether. It is one the earlier steps in the reaction mechanism resulting in the octaethylene glycol derivative of 1,1,1,3,5,5,5-heptamethyltrisiloxane. Such an initial Williamson synthesis reaction had to be carried out so that later steps in the reaction—that is, ones involving material types not readily accessible—could successfully yield the derivative product. The resultant glycol derivative is an example of a defined surfactant. This particular journal article focused on the correlation between surfactant constituents and the effect on properties such as spreading performance.
The Williamson synthesis involves an SN2 reaction in which a halogen, sulfonyl, or sulfate group is replaced by an alkoxide ion, which can itself be prepared by a reaction of the alcohol with an active metal such as sodium or its hydride (i.e. NaH). The resultant alkoxide salt then reacts with the alkyl halide (must be primary) to produce an ether via the SN2 mechanism.
Other examples of Williamson synthesis of ethers can be found in this same reaction mechanism used to produce the surfactant. Article Link.
Preparation of Progesterone from Cholesterol chymitry
This is what I understand happens, following the process of production of progesterone, described on page 894, second column.
In the first two steps cholesterol is brominated in benzene, and oxidized in a solvent with acid permanganate(aq). In the last step the product is again debrominated using zinc dust.
The strong oxidizing agent potassium permanganate is used, as well as sulfuric acid. The article below describes the process of production of progesterone from cholesterol.
Progesterone has numerous physiological effects. Although primarily associated with the reproductive system, progesterone has multiple effects outside of it. This steroid hormone can act as an antiinflamatory agent, reducing the immune response; it can also assist in thyroid hormone action and bone building. Progesterone appears to prevent endometrial cancer (cancer involving the uterine lining) as well as breast cancer (1).
Article link
Catalytic Hydrogenation of an Acid chymistry
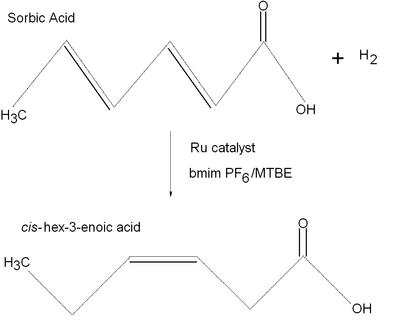
This reaction is an example of the catalytic hydrogenation of an acid in an ionic liquid similar to the reagents sodium metal/liquid ammonia discussed in lecture. This particular reaction has sorbic acid and hydrogen gas reacting with a ruthenium catalyst and a biphasic 1-butyl-3-methyl imidazolium hexafluorophosphate (bmimPF6)/methyl tert-butyl ether (MTBE) system to create cis-hex-3-enoic acid. The above reaction occurs with ~85% selectivity. The author of this paper was examining enantioselective hydrogenation in ionic liquids because this mechanism could provide a means for facile recycling of metal complexes of expensive chiral ligands. The author also studies some hydrogenation reactions that lead to the precursor of the antiinflammatory drug ibuprofen, the active ingredient in Advil.
For the full text click here.
Production of Hydrogen by means of hydrogenation of ethanol chymitry
Along with the growing interest in Fuel Cell powered cars comes the need for higher production methods of Hydrogen, both in bulk form and in-car conversions (for fuels such as methanol and ethanol to be converted to hydrogen on board). Previous methods of converting Ethanol to hydrogen was by means of high-temperature steam reformation (at temperatures in excess of 600° C) to produce Hydrogen gas and CO.
This journal describes a special method of low-temperature dehydrogenation of ethanol over special Raney catalyst with Cu added to it. The first step produces one mole each of hydrogen gas and acetaldehyde (per mole of ethanol). This is followed by the decarbonylation of acetaldehyde to form methanol and CO. The whole reaction undergoes a water-gas shift to net one mole each of Methane and Carbon dioxide and two moles of Hydrogen.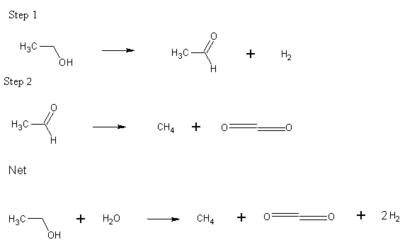
Compared to high temperature reformation methods, which produces 6 moles of hydrogen per mole of ethanol, this reaction doesn't seem as fuel efficient, though the authors were confident, that with an internal combustion engine on-board that uses the methane produced as fuel, the total output energy would be equal.
Citeulike Link
Synthesis of Lupinine Esters from Betulonic Acid Chloride chymistry
The reaction shown above outlines the synthesis of the lupinine ester. These esters have been studied for their biological properties. Found mainly in plants there is heavy research being done on them for their antiviral, antitumor and hepatoprotective activity. In some cases lupinine esters can exhibit local anesthetic properties.
The reaction shows how you would synthesize a lupinine ester from betulonic acid chloride with lupinine. Side conditions for this reaction include the presence of triethylamine and must be performed in dry CCl4.
CiteULike Reference
Second deadline over chymistry
All of the grades for the second blog assignment deadline are now posted and cannot be changed. A few comments:
1) Most of you are now using CiteULike properly so there are now plenty of examples other students can look at to see how it should be done.
2) It would be nice if you could tag all your articles with "orgo" so that all the articles of the class could be tracked with an RSS feed.
3) Many of you are giving a lot more than what I am asking for an running into some trouble in the process. All I want is one single reaction with nice readable molecules drawn from ChemSketch (or by hand as a last resort) with an arrow and reagent on the arrow. It will be easiest for you to pick a reaction that we covered in class because you won't have to explain it in as much detail.
4) If you want to give me more information that is fine but make sure that you support your statements with a link to a document with primary data (like experimental results) or to a document where the information trail continues on to primary data. For example, "progesterone reduces the risk of cancer" should be clickable to an article where the actual study has this as a conclusion OR to an article that has a similar statement with a link to primary data. References lumped at the end of your post are not good enough. Articles that you link to with general references at the bottom are also not usable. Unfortunately this means that a lot of government public health sites or company websites are not suitable for references. Your opinions and your speculations don't have to be supported by a reference. Try to post well before the next deadline so I can help you get this right.
ChemSketch Demo chymistry
Here is a 3 minute Flash screencast that Yana made to help with the use of ChemSketch in your blog assignment. If you know how to do something well please consider doing a brief screencast like this. You can download Camtasia here.
Even if something seems simple to you, it may not be for everyone. A good way to know if a screencast it useful is to notice recurring errors made by student in their blog assignment.
Examples:
-how to find a reaction in the literature
-how to use CiteULike
-how to post to Blogger
-how to resize pictures
-how to link to a reference without pasting the entire url in the post text
-how to tell if a reference is suitable
etc.
Production of ketone from an alcohol: an unwanted side product chymistry

The above reaction shows an epimeric steroid alcohol being converted by a catalyst of ruthenium and aluminum oxide to a racemic mixture of 17-estradiol 3-methyl ether and a ketone. The racemic mixture is formed by converting the beta version of the ether, where the hydroxyl group is on the top of the ring, to the alpha version of the ether, where the hydroxyl group is underneath the ring. The reaction is stopped when the amount of alpha ether is roughly equivalent to the amount of beta ether. In the notation used, the wavy line between the hydroxyl group and the ether shows that the hydroxyl group can be in either the front or the back of the molecule.
However, a ketone can be produced instead of the alpha ether when the alcohol is oxidized. Because the purpose of the reaction is to racemize the ether, this ketone is an unwanted side product. To prevent oxidation, toluene at 100 C is used as a solvent. The chemical properties of toluene slow the formation of the ketone so that at temperatures around 100 C, the yield of the racemic mixture is about 54%. Any ketone that does form can be separated from the ether by flash chromatography. This reaction is a good way to racemize the ether efficiently and inexpensively; it was traditionally synthesized at a much higher cost.
For the full text of the article describing this process, see this report.
Cesium fluoride-Celite: a solid base for efficient syntheses of aromatic esters and ethers chymistry
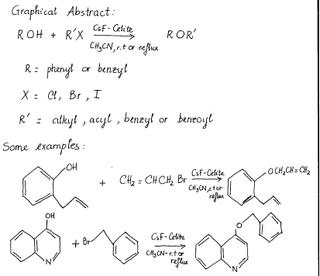
In the syntheses of aromatic esters and ethers, CsF-Celite has been found to be a very efficient, convenient and practical reagent. In fact, it is used for the coupling reactions of a number of aromatic and heteroaromatic phenols with alkyl, acyl or benzoyl halides.
Many other organic reactions have recently been catalyzed by CsF-Celite, such as the reactions to synthesize carboxylic esters, γ-lactones, N-alkylation of anilines, or carboxamides.
For more reference, please click on http://www.citeulike.org/user/hongan1985/article/270811
Play for points chymistry
The final deadline for the 4th blog assignment is August 19th. Since you have had the benefit of my feedback for the first 3 assignments you should now be able to do this one without feedback or problems. It is worth 2% of your grade.
As an alternative to this final blog assignment you may volunteer to evaluate the Unreal Tournament maps we have available on the class material. The deadline for this is also August 19th. Don't wait too long to sign up for this because it will require scheduling. If you don't have UT I'll give you access to a computer.
Contact me if you are interested.
Anaerobic Toluene Oxidation in the Metabolism of the Fe(III)-Reducing Microorganism GS-15 chymistry
This is a potential pathway for the oxidation of toluene in Fe(III)-reducing microorganisms, which play important roles in sediments naturally composed of hydrocarbons. The oxidation of toluene, an aromatic hydrocarbon, in these microorganisms is coupled to Fe(III) reduction. GS-15 is the first microorganism discovered to link aromatic compound oxidation to the reduction of Fe(III). The oxidation of p-cresol and phenol in these organisms is also coupled to Fe(III) reduction. Under strict anaerobic conditions in these organisms, GS-15 can completely oxidize toluene to carbon dioxide by utilizing Fe(III) as the only electron acceptor in the reaction.
This mechanism can be used to clean up toxic oil spills or other toluene contaminations by introducing the microorganisms to the site.
Here is the link to the CiteULike article discussing these anaerobic metabolic processes.
Reduction of Aldehyde to an Alcohol -- Synthesis of D,L-1,2,4-butanetriol chymistry
D,L-1,2,4-butanetriol can be made in two different ways; the first way is commercial synthesis through reduction of esterified D,L-malic acid with sodium borohydride, NaBH4, while the second way involves microbes. The latter method was the focus of the journal article. Nitration of racemic D,L-1,2,4-butanetriol results in D,L-1,2,4-butanetriol trinitrate, a compound that is the energetic equivalent of nitroglycerin, but is less shock sensitive, more thermally stable, and less volatile. One of the final steps in the synthesis of D, L-1,2,4-butanetriol via microbes is the reduction of a racemic mixture of D,L-3,4-dihydroxybutanal (aldehyde), to the final alcohol product, as seen in the reaction below. The catalyst for the reaction is dehydrogenase from E. coli.
For the full text of this article, click on the following link (and then the second listed link in citeulike):
Article Link.
Synthesis of Phosphate Monoesters chymistry
The following paper presents an efficient and environmentally friendly method for producing phosphate monoesters, in which the only byproduct is water, as opposed to a different reaction, which also gives off pyridine hydrochloride as a byproduct.
The paper describes that it was experimentally determined that the best solvent, tertiary amine, and catalyst (or nucleophilic base) for the reaction are DMF-nitroethane, tributylamine, and N-butylimidazole, respectively, each giving the highest yield. This is a summary of the main reaction discussed in the paper.
* The article states that water was constantly removed by azeotropic reflux, so that the reverse reaction was prevented.
Article Link
I have also included an interesting extensive study on phosphate monoesters.
Synthesis of S-Adenosylmethionine Synthetase chymistry
The sulfonium salt S-adenosylmethionine is one of the most widely used biological methylating agents. It is formed by the ATP activation of methionine. One of the most benifical uses of this salt is to convert norepinephrine to epinephrine (adrenaline). Many times this conversion happens in flight or fight organs, which leads to vasodilatation. This vasodilatation in turn leads to an increased blood flow to the organ/tissue or interest.
The reaction shown is the formation of S-Adenosylmethionine. This process occurs in two steps as can be seen. The first step cleaves the whole phosphate of the ATP. However before the sulfur of methionine attacks the C5` atom of ATP (via SN2) there is further hydrolysis of the cleaved tri-phosphate into two inorganic phosphates (di and mono).
Here's a link
Chemistry of a Breathalyzer chymitry
A Breathalyzer makes use of the fact that alcohols (in this case ethanol) oxidize into carboxylic acids. It uses the strong oxidizing agent Potassium dichromate in a yellow solution of sulfuric acid, under the presence of a Silver Nitrate catalyst, to complete the reaction quickly. As ethanol oxidizes and the Potassium dichromate reacts, the chromate ion changes from Cr (VI) to Cr (III). This causes the color intensity of the yellow solution to decrease, and a spectrophotometer in the breathalyzer compares the absorbance of this solution with that of an unreacted solution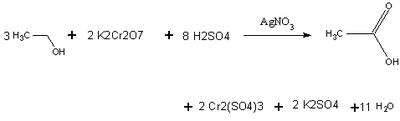
Using Beer's law, the spectrophotometer can relate concentration to absorbance levels of the chromium ion. The amount of alcohol present is proportional to the stoichiometric coefficients. An actual breathalyzer only needs to detect 25 micrograms of ethanol to give a reading 0.10 Blood Alcohol Level.
Journal of Chemical Education
Asymmetric synthesis of monohydroxy tetradecanoic acids chymistry
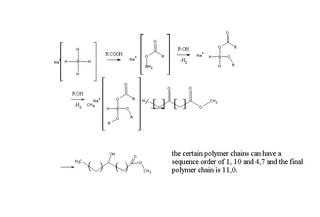
Methyl 3-, 6- and 13-oxo tetradecanoates went through reduction with NaBH4 in the presence of 1,2:5,6-di-O-isopropylidene-Dglucofuranose (DIPGH) and menthol together with isovaleric and pivalic acids in THF solution. This work signifies the importance of positional
effect. The position of lower steric hinderance and higher enantiomeric excess and asymmetric reduction yield were noted down, namely the prochiral 13-keto isomer structures.
With this asymmetric reduction at normal atmospheric pressure together with inexpensive auxiliaries make it competitive with other reduction methods and is needed to assess the need in the market. Here's a link for this article
Benzoylation of a Polyol chymistry

Amino polyols are an important part of synthetically created amino acids. They are highly antibacterial and immunosuppressive and so are used in various antibiotics and antifungal products. This reaction shows one of the step necessary in creating the amino polyol.
Benzoyl chloride is added to the polyol to form a tribenzoate compound. In this reaction, the polyol has several R-O-H groups that act as weak nucleophiles. When the benzoyl-Cl bond breaks upon addition to the pyridine solvent, the benzoyl group acts as an electrophile. With the help of the DMAP (dimethylaminopyridine) catalyst in the reaction, the R-O-H group is deprotonated and the benzoyl group is added to the remaining R-O form the final tribenzoylated product. As seen above, the reaction has a yield of about 90%. The OTBS (t-buytldimethylsiloxy) groups do not participate in this reaction.
There is one hydroxyl group left on the molecule produced. All of the hydroxyl groups would be replaced by benzoyl groups if the reaction was not stopped after three groups had been added. To stop the reaction at this point, three equivalents of benzoyl chloride were used for every polyol.
For the full text of the article describing this process, see this report.
Zinc reduction of alkynes chymistry
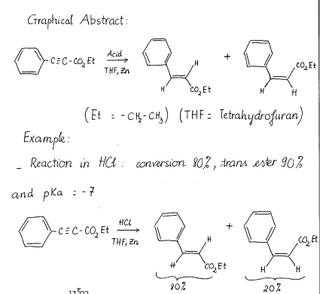
A traditional method for the reduction of alkynes to trans-alkenes is to dissolve metal reduction using sodium or lithium in ammonia. However, we can also use Zinc as a metal to reduce alkynes.
In this specific case, by changing the proton source in the reaction, the dissolving Zinc metal reduction of ethyl phenylpropiolate to the corresponding cinnamate ester can be stereochemically controlled.
The product of the reaction will be a mixture of cis and trans ester. By this reaction, we can see the efficiency of Zinc in the reduction of alkynes.
For more reference, please click on:
http://www.citeulike.org/user/hongan1985/article/279061
Diels-Alder reaction of Quinol Lactone chymistry
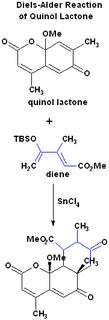
This is a Diels-Alder reaction, which does not follow the regiospecificity rules. What makes this reaction unusual is that normally quinol-lactone does not react with dienes. Also if we follow the regiospecificity rules we would logically expect to get the structure in Figure 2. However by using stannic chloride (SnCl4) in methylene chloride, the product shown was obtained. It was determined through NMR spectroscopy that this particular product was present, and not its isomer in Figure 2. TBSO stands for the tert-butyldimethylsilyl group.
The reaction is described in this article.
I found an article which looks more closely at the process of migration and elimination of TBS when the final product is synthesized, which produces TBSOTf. It states that stannic chloride causes the migration of the tosyl group (1st paragraph of "Results and Discussion" section). I believe this plays a role in the final "inversion" of the product in the posted reaction. Also this Diels-Alder reaction is performed in Lewis acid which gives the regioselectivity opposite to what we would expect in an uncatalyzed reaction.
The product in Figure 2 should be expected, because if we examine the ortho-para rules for regioselectivity through the formation of radicals without a catalysit (Lewis acid), we can see that the major product should resemble the one in Figure 2. There is an example on this page under the "Regioselectivity" section, which has the major product boxed in.
Free-Radical Polymerization: Alkoxyamine Initiators chymistry
Polymers have important uses in both research and industry. Alkoxyamines are used in ATRP (atom transfer radical polymerization)-based polymerizations and can serve as efficient regulators in the preparation of polymers. In the past, the alkoxyamines were produced by the creation of radicals that are carbon-centered and were then trapped by nitroxide. This method, however, gave low yields and undesired byproducts. The reaction shown here takes place at low temperatures and in the presence of a nitroxide, utilizing an ATRP-based initiator that is treated with copper bromide. The ATRP is involved in the living radical polymerization system. Me6-tren ligand forms a catalyst complex for the reaction of the initiator with nitroxide. Equilibrium between the transfer to and from radicals and dormant species in the reaction is controlled by the Me6-tren ligand forming a complex with the Cu(II), which the free radicals can then interact with. The catalyst name Me6-tren stands for the chemical tris(2-(dimethylamino)ethyl)-amine. This is a more effective procedure for preparation that results in high yields. Discoveries such as this are important in areas such as nanotechnology.
Here is the CiteULike article.
Here are articles about the Me6-tren ligand and its naming.
This source discusses the use of alkoxyamines in free-radical polymerization.
Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility chemistry
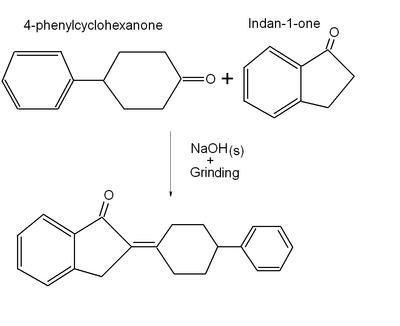
Recently there has been a paradigm shift away from using solvents in organic synthesis as solventless reactions can lead to improved outcomes, and more benign synthetic procedures, in for example an aldol condensation reaction as shown above. Sustainability is increasingly an important issue in broader context when you are talking about health, energy, and the sciences. Removing organic solvents in chemical synthesis is important in the drive towards benign chemical technologies. Organic solvents are high on the list of toxic compounds due to the problems in containing volatile compounds and the sheer large volume of them used in industry.
Some advantages of utilizing solventless reactions are that the compounds are often sufficiently pure to avoid extensive purification using chromatography, the reactions can be rapid, often reaching substantial completion in several minutes compared to hours in organic solvents, and the energy usage can be much lower.
For the full text click here.
Bimolecular Dehydration of 1-Pentanol to Di-n Pentyl Ether (DNPE) chemistry
Regulations controlling diesel exhaust become more exacting with each passing year. Accordingly, diesel fuel properties are constantly being analyzed in an attempt to further reduce fuel emissions. There are many options, most often refinement processes or improving the cetane number. Essentially, short and branched ethers (used in gasoline) have a good octane number but poor cetane number, while those ethers used in diesel are linear and have a comparatively long chain (ideally 9 or more carbons). Di-n pentyl ether (DNPE) has shown most effective in reducing emissions, and is also relatively simple to synthesize via the bimolecular dehydration of 1-pentanol on acid catalysts, as seen below.
However, the dehydration reaction results in quite a lot of byproducts, including other ethers. As such, a selective catalyst is required to favor production of DNPE by reducing the amount of alkenes. Increased selectivity can be accomplished via gel-type acidic resins at a reaction temperature of 150°C. The article I looked at analyzed the selectivity and reaction rate of the dehydration of 1-pentanol to DNPE using a gel-type resin at various temperatures and alcohol flow rates. Article Link.
Last Blog Assignment Deadline Over chemistry
All grades for the last blog assignment have been posted. It is now too late to add or change your posts.
I have been impressed with many of the posts. Most of you found relevant articles and extracted the information you needed. Several of the posts were about very practical things like environmentally friendly synthetic approaches, alternative fuels, breathalizer chemistry and the use of enzymes and biological agents to clean up pollutants.
One of the reasons I started this blog assignment is to help you understand how the reactions you are learning are used by chemists in the real world of research and industry. A side benefit is that I have learned some chemistry on each and every post. As a chemist you will never stop learning. What I am hoping is that in this class you have learned the skills to find the information you need to use chemistry productively in your career.
Another bonus is that other people from around the world are benefiting from your posts. Click on the SiteMeter button at the bottom of the blog then click on referrals to see how people are finding your posts.
I will be cleaning up the blog at the end of the term, removing unreadable or substantially incorrect posts. If you wish to keep your posts make sure to copy them before then. In Blogger you can copy a post from one blog to another easily. Just make sure you copy and paste in the "Edit HTML" view, not "Compose". Note that you can also convert the posts into Word. You may wish to do this also if you want to build an e-portfolio.
Welcome Winter 2006 class chemistry
Your starting point for this class is the wiki. I will use the blog this term only for announcements and this is where your extra credit blogging assignments get posted. See the assignments from previous terms in prior posts here and look at the comments to see my feedback and grading system.
First week update chemistry
1) Students who came to the workshops got all of their technical issues squared away and we worked on alkyne problems. A few students also worked on their extra credit assignments.
2) You should all have completed lecture 3 by this point (alkynes). If you are having any technical problems it is imperative that you deal with this at the next workshop on Wednesday.
3) We ran into a few glitches this week with accessing the Real Player lecture recordings but I posted a workaround on the wiki so that you would still have access through the guest account. Right now the Real Player lecture archive in WebCT is working fine. The PDFs associated with some of the lectures have been moved to Item 11 on the wiki. Not all the lectures are there yet but the ones for the first week are and the rest will be there shortly.
second week update chemistry
Just a reminder that you should now be at the end of Chapter 10 problems and quiz and have had any confusion resolved at the workshops or by emailing me. You should have all technical issues addressed by now.
The first test is coming up in 2.5 weeks.
Remember that the Friday workshops are in Matheson 109.
Class Vodcast available chemistry
Subscribe to the class screencasts on your video ipod by dragging the vodcast icon (from the class blog) into iTunes. Even without an ipod, you can view the screencasts from iTunes on your computer.
The quality is not as good as the Flash screencasts but it should be usable for the most part. Let me know how you find it.
The first 2 weeks are up now - more to follow.
Powerpoint and Problem set downloading chemistry
There seems to be a problem with downloading files from WebCT Vista.
You may now download the Powerpoints and the problem set pdf off of the class wiki (Items 4 and 12)
no workshop Jan 27 chemistry
There will be no workshop tomorrow Friday Jan 27, 2006. We'll resume on Monday.
Race on Monday - try to win an ipod chemistry
There will be an Edufrag Race using the educational version of Unreal Tournament on Monday Feb 13, 2006 during our workshop time at 9:00 in CAT 061. There will be 20 rooms in the maze on any topic from CHEM 241 and up to test 1 in CHEM 242.
I may include contributions from students in this class so this might be a good time to work on your extra credit due by Feb 17th.
The first student to make to the catroom has an equal chance of winning one of the following:
- 30Gig video ipod
- molecular model kit
- book
- consolation mystery prize
Hock Process in the Manufacture of Phenol chemistry
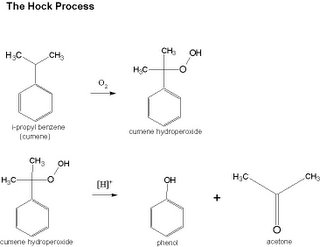
Phenol is more important than most people realize. It can be found in many consumer products including aspirin, head lights, gas tanks, billiard balls, nylon, wintergreen gums, Pepto Bismol, deodorant and more. A side product in the manufacture of phenol is acetone which is also used in the private sector in plastics, solvents, and more.
The high-yield manufacture of phenol uses the concepts of peroxidation and cleavage. Cumene (i-propyl benzene) is oxidized by exposure to air to temporarily produce cumene hydroperoxide. The cumene hydroperoxide is simply cleaved at the top of the benzene ring using an acidic catalyst to produce the two usable products of phenol and acetone. The catalyst is extracted and the phenol/acetone mixture is fractionated and purified. Under optimal conditions, 1.31 tons of i-propyl benzene (cumene) will produce 1 ton of phenol and 0.615 tons of acetone. The end-product phenol purity is at 99.99 wt % with total impurities of only 60 ppm. This process is termed the Hock process after being discovered by Hock and Lang in 1944. This process was ideal since both products were useful and relatively pure. Modern demand, however, for phenol is increasing at a higher rate than acetone. This means that the future may classify acetone as a partial waste product. More information on the Hock Process of manufacturing Phenol can be found here which expands on the Benzene-Free Synthesis of Phenol or here which discusses Selective decomposition of cumene hydroperoxide into phenol and acetone by a novel cesium substituted heteropolyacid on clay.
Feb 13 2006 Race chemistry
Today's edufrag race is available for download here.
Nick was the winner in 6 minutes. He drew the consolation prize (cat keychain). The ipod video will be up for grabs at the next race - we'll try for Friday.
Fischer Esterification: 2-ethylhexyl benzoate chemistry
Fischer esterification can be a time-consuming process, requiring days for a reaction to reach equilibrium. In this article, researchers developed a way to hasten this process by using a specially designed microwave to heat the reaction quickly and evenly and at an increased pressure. In order to test the efficiency of the device, they synthesized 2-ethylhexyl benzoate from benzoic acid and 2-ethylhexanol as shown below.
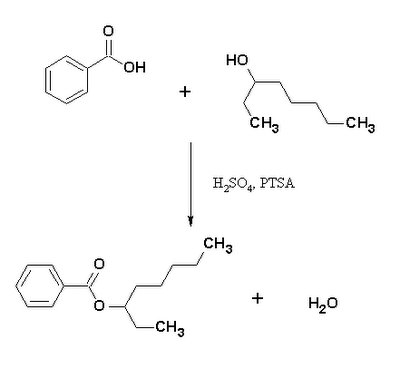
Sulfuric acid as well as para-toluene sulfonic acid (PTSA) were used to catalyze the reaction. In order to shift the reaction towards the products, a large excess of 2-ethylhexanol was used and the water produced was constantly removed. One of the disadvantages of Fischer esterification is that dehydration can also occur, resulting in unwanted ether and alkene products. Because of this, the temperature and catalyst concentration must be carefully monitored. The researchers were able to show that microwave heating causes no ill effects on the reaction and reduces the time required to a matter of minutes while still producing a high level of the desired product.
Feb 17 2006 race chemistry
This morning's Unreal Tournament race was won by Raymond in 8 minutes.
He won a book - the video ipod is still up for grabs.
Attend the next race Friday at 10:00 in Matheson 109 for a chance to win it.
next workshop chemistry
There will be no workshops this week until Monday Feb 27, 2006.
We'll also have another Unreal Tournament race on that day, covering the basics from CHEM241 and possibly anything in CHEM 242 up to and including NMR. The video ipod is still available as a prize.
Of course, I am also there to help with the class material, as usual. Generally students find NMR to be a little harder than the rest of the material so don't procrastinate coming to the workshops for help with that.
Feb 27 race chemistry
Monday's edufrag race was won by Nick again. He drew the book prize.
The ipod video will be up for grabs at the next race - lets shoot for Monday March 6th for the next race.
I would like to include some NMR in the next race so if you were thinking of doing your extra credit on UT doors, this would be a good time to work on it.
Also note that I can help you with ANY class topic at the workshops.
iPod won today chemistry
Louie won the video ipod in this morning's race in an impressive 4 minutes.
I think all of the NMR concepts we covered in this class are in there so you may want to try this one to practice for the test.
Lets do another race on Friday, although I can't promise there will be another ipod available.
frustrated winner chemistry
Nick won this morning's race in 10 minutes. This is his third win and he drew the molecular model set. The only prize left for him to win is the video ipod, since I don't give out the same prize twice to the same person. We'll do the final race of the term on Friday March 17, 2006.
This maze is all about IR and NMR and is a good way to review the material for the test and exam.
Exam Quiet Time Reservations chemistry
Sunday March 19th Room B-103 3:00-9:00 pm
Tuesday March 21st Room C-104 3:00-9:00 pm
Room B-103 has been reserved on Monday March 20th and Wednesday March 22nd from
4-9 pm
The Chemistry Behind Lightsticks chemistry
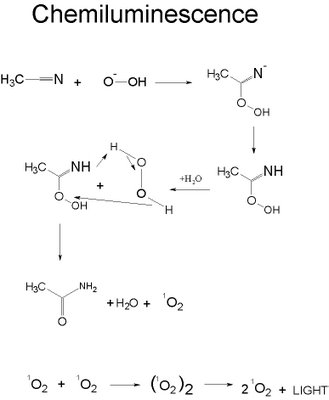
Almost everyone has seen a lightstick. A lightstick is a plastic tube with a glass vile inside it. When the tube is bent, the vial breaks allowing the chemicals to mix and react. The colorfully glowing sticks utilize a chemical process called chemiluminescence where energy is released in the form of light. The most common lightsticks use chemiluminescence with colored tubes to provide the desired color.
This process is not caused by heat and may not produce heat, but the speed of reaction is still dependence on environmental heat. The colder the environment, the slower the reaction and will glow longer.
Lightsticks have three parts. There are two chemicals that react to release energy which is converted to light. Usually, commercial lightsticks utilize the reaction between hydrogen peroxide and acetonitrile. When the glass vile is broken and the two chemicals are mixed, it will release enough energy to excite the electrons in the oxygen to cause the electrons to jump to a higher energy level and then fall back releasing light.
Specifically, the hydrogen peroxide oxidizes the acetonitril eventually forming excited oxygen. This decomposes and releases the energy as light as can be seen stepwise above.
More on chemiluminescence can be found here on “A Chemiluminescence Reaction between Hydrogen Peroxide and Acetonitrile and Its Applications.”
The last race chemistry
Monday, July 14, 2008
YACB - Yet Another Chem Blog
I need to do a blog like I need another hole in my head. Does the world really need another chemistry blog? Probably not. So, why am I doing this? I have no idea. I guess because it's fun? There's so much science out there that seems very impersonal. Perhaps blogging about chemistry allows us to inject our personal views and commentary on current chemistry. My hope is that this blog allows me to present what I find interesting in the world of Organic Chemistry and hopefully inspires people to pursue this wonderful scientific discipline.
ASAP Thursday
The first thing I do when I get into the lab in the morning is make my coffee. The second thing is to see what has appeared on the web journals. I suppose since I'm blogging that I should share with you the articles that catch my eye. Here's a couple from today.
Karl Scheidt has a very nice example of umpolung chemistry catalyzed by N-heterocyclic carbenes in a [3+3] cycloaddition. This appeared on the web yesterday.
Audrey Chan and Karl A. Scheidt, JACS DOI: 10.1021/ja0709167
Interestingly, I was just teaching my synthesis students about the utility of diazo compounds for cyclopropanation and Wolff rearrangements and what appears on OL this morning? A very nice and practical method for the preparation of diazo compounds. This could come in handy.
Muhammad I. Javed and Matthias Brewer, OL DOI: 10.1021/ol070515w
Feline Frolics
I have two cats and they drive me absolutely nuts. Always demanding and always getting in trouble. Today was no different. I had need of some thyme for my spice cupboard, so I snuck out at lunch and stopped by my favorite health food store that has a huge array of dried herbs and spices in bulk. I bought an ounce of thyme and some other goodies and stopped off at home to put them away. No sooner did I drop the bag on the kitchen floor and head to the 'little chemists room' did my biggest pain in the ass, Sam, discover a new toy. Yes, a small little plastic bag of thyme. By the time I got back to the kitchen, he had ripped the bag open and was squirming around on the floor in a big mess of herbs! *sigh* You'd think it was catnip or something, the way he was carrying on. Out of curiosity, I dug up information on the compound found in catnip. It turns out to be nepetalactone. Nothing I could find indicated that thyme contains this terpene. So what was Sam all worked up about? Well, the major volatile constituent of thyme is the terpene thymol. Very different structure than nepetalactone. Although thyme does not contain nepetalactone, catnip does contain significant amounds of thymol. Interestingly, thymol is also used as in ingredient to repel feral cats. Sam sure is odd.

Mechanism Challenge Answered
Tynchtyk, over at Chemist in a Transition State, posted a very interesting transformation and challenged us to propose a mechanism. Here is the reaction. At first glance, this looks like some kind of reductive amination reaction. However, on closer inspection, you can see that there is one less carbon in the product than the starting material. Furthermore, there are no reducing agents present, only acid (and presumably water). Of course the obvious starting point is to react the secondary amine with the aldehyde to form a cyclic imminium structure. Once generated, this is nicely set up to undergo a [3,3]-sigmatropic rearrangement to transfer an allyl group to the imminium carbon. The resulting formaldehyde imminium product is then hydrolyzed in the presence of water to afford the product plus an equivalent of formaldehyde. The full mechanism is shown below. Notice I am a stickler for showing every proton transfer step! No shortcuts here.
At first glance, this looks like some kind of reductive amination reaction. However, on closer inspection, you can see that there is one less carbon in the product than the starting material. Furthermore, there are no reducing agents present, only acid (and presumably water). Of course the obvious starting point is to react the secondary amine with the aldehyde to form a cyclic imminium structure. Once generated, this is nicely set up to undergo a [3,3]-sigmatropic rearrangement to transfer an allyl group to the imminium carbon. The resulting formaldehyde imminium product is then hydrolyzed in the presence of water to afford the product plus an equivalent of formaldehyde. The full mechanism is shown below. Notice I am a stickler for showing every proton transfer step! No shortcuts here. Tynchtyk says this problem appeared in a science olypiad for High School Students in Moscow. I wish our high school education here in the states was up to this kind of challenge.
Tynchtyk says this problem appeared in a science olypiad for High School Students in Moscow. I wish our high school education here in the states was up to this kind of challenge.
Thanks, Tynchtyk, nice problem! In the spirit of problem solving, let me pose a new challenge. This is one of my favorite transformations.
Beta Amino Acid Rearrangement
Here's the answer to the mechanism question I posed at the end of the last post. Some have suggested a 4-membered ring intermediate. While that cannot be ruled out, a mechanism that does not include the high strain of a bridged 4-membered ring seems more plausible.
Since this is an aminoacid, it will exist in it's zwitterionic form. Thus, the quaternary ammonium will not be acylated. The carboxylate is converted to a mixed anhydride. Then it undergoes a beta-elimination of the ammonium to open the 6-membered ring. This is followed by an acylation of the resulting amine to form the rearranged lactam. This reaction was reported by Henry Rapoport (JACS 1970, 92, 5781). He cites an older paper by Ferles (Coll. Czech. Chem. Commun., 1964, 29, 2323.
This reaction was reported by Henry Rapoport (JACS 1970, 92, 5781). He cites an older paper by Ferles (Coll. Czech. Chem. Commun., 1964, 29, 2323.
Update: As liquidcarbon points out in the comments, the free amine of the ring-opened intermediate above would likely be acetylated in refluxing acetic anhydride. Another possible route to the product would involve an intramolecular acylation forming a bridging 4-membered ring, followed by beta elimination. Possible, but I'm not sure how well the bridgehead hydrogen sigma orbital would overlap with the sigma star orbital of the C-N bond.
ASAP Thursday
Some good papers have shown up on ASAP this week. Here's two that rose to the top for me.
First is a contribution from Shu Kobayashi with some very interesting chemistry using chiral Calcium complexes. The reaction he investigated was the Michael addition of glycine derivatives with acrylates. He showed the importance of an enolizable proton on the bis-oxazoline ligand and suggests that the reactive species is a calcium Brønsted base. This generates a chiral calcium enolate that undergoes Michael addition to the acceptor. Subsequently, an intramolecular Mannich reaction ensues to afford pyrrolidines in very high selectivity.
Susumu Saito, Tetsu Tsubogo, and Shu Kobayahsi, JACS, DOI: 10.1021/ja0709730 Organocatalysis is all the rage now, and even we are trying our hand at some. Xiao has just reported a slightly new twist on organocatalysis by carrying out an intramolecular Friedel-Crafts reaction with indoles to form tricyclic compounds. Selectivities are are pretty good in some cases.
Organocatalysis is all the rage now, and even we are trying our hand at some. Xiao has just reported a slightly new twist on organocatalysis by carrying out an intramolecular Friedel-Crafts reaction with indoles to form tricyclic compounds. Selectivities are are pretty good in some cases.
Chang-Feng Li, Hiu Liu, Jie Liao, Yi-Ju Cao, Xiao-Peng Liu, and Wen-Jing Xiao, OL, DOI: 10.1021/ol0703130
Cross Coupling of Anilines
A fascinating paper appeared on the JACS ASAP site this morning from Ueno, Chatani and Kakiuchi. They used a ruthenium catalyst to carry out a cross coupling of an aryl amine with a phenyl boronate. What is remarkable is the fact that the transition metal did oxidative addition to an aryl-nitrogen bond. Success of the reaction was dependent on having a chelating carbonyl group adjacent to the amine, however, this is still the first example of oxidative addition to a hitherto unreactive C-N bond.
Satoshi Ueno, Naoto Chatani, and Fumitoshi Kakiuchi, DOI: 10.1021/ja0713431
A Rhodium Thing
Ever since my first encounter with aldehyde C-H insertion by rhodium, I have been intrigued by the possibilities of the acylorganometallic intermediates. In Org. Lett. a nice formal [4+2] cycloaddition appeared utilizing ortho-vinyl benazaldehydes and olefins or alkynes. The reaction proceeds via C-H insertion to form an acylrhodium followed by a migratory insertion to produce a rhodacylopentene. This reacts with an alkene to form the product shown. Chiral ferrocenyl phosphine ligands afforded at least modest level of enationselectivity. If alkynes were utilized, napthol products were produced. Although most yields and selectivities were modest, it is an interesting transformation. It seems to be limited in scope. Without the arene ring cyclization did not take place. Instead the acylrhodium simply reacts with the olefin in a reductive Heck-type process to afford an acyclic ketone.
Ken Tanaka, Daiki Hojo, Takeaki Shoji, Yuji Hagiwara, and Masao Hirano, DOI: 10.1021/ol0704587
Your Road to a PhD
 I'd like to call out some of the lurkers who read my blog to come forward and make some comments. I'm curious to find out what influences a student's decision for choice of graduate school in chemistry. So, if you have your PhD, are in a PhD program now, or going to join a PhD program soon, please tell me what was important for your choice of school. Was it location? Science? Stipend? What? Did family issues change your decision? Would you only look at schools on the coasts, or did you look across the middle of the country too? How influential were your undergraduate mentors? What about international studies? This would apply to those from the US going abroad as well as those from outside the US coming to America. And finally, what sources did you use to find graduate programs? Do you put any stock in the web sites that list rankings of graduate programs?
I'd like to call out some of the lurkers who read my blog to come forward and make some comments. I'm curious to find out what influences a student's decision for choice of graduate school in chemistry. So, if you have your PhD, are in a PhD program now, or going to join a PhD program soon, please tell me what was important for your choice of school. Was it location? Science? Stipend? What? Did family issues change your decision? Would you only look at schools on the coasts, or did you look across the middle of the country too? How influential were your undergraduate mentors? What about international studies? This would apply to those from the US going abroad as well as those from outside the US coming to America. And finally, what sources did you use to find graduate programs? Do you put any stock in the web sites that list rankings of graduate programs?
Odeur d'Asperge
Yes, it is asparagus season. I love this vegetable. Raw straight out of the garden, steamed with white wine and garlic, coated with olive oil and roasted on the grill, I can't get enough of it! Of course, for some of us that means plenty of malodorous urinary discharge. Yes, I'm talking about the infamous Asparagus Pee! The culprit - Asparagusic Acid. Derived from valine, this acid is unique to asparagus and appears to be the metabolic precursor to a number of odiferous sulfur-containing compounds. In it's pure form, this colorless solid melts around 76 °C. For those with the enzyme to break it down (~40% of the population) the results appear in the urine usually within 15 minutes of ingestion. Interestingly, not everyone has the ability to smell the satisfying stench, thus complicating studies to determine why some have smelly piss and others do not. I am one of the lucky ones who can both produce copious quantities of metabolites such as methyl thioacrylate (among others) and delight in its pleasing perfume. For those of you deprived of this evolutionary gift, I have sympathy, as you will never truly enjoy the full experience of asparagus season.
(edited 5/8/07, 5:28 pm to fix structures)