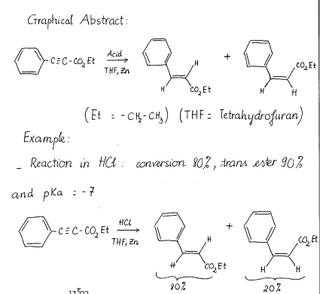
A traditional method for the reduction of alkynes to trans-alkenes is to dissolve metal reduction using sodium or lithium in ammonia. However, we can also use Zinc as a metal to reduce alkynes.
In this specific case, by changing the proton source in the reaction, the dissolving Zinc metal reduction of ethyl phenylpropiolate to the corresponding cinnamate ester can be stereochemically controlled.
The product of the reaction will be a mixture of cis and trans ester. By this reaction, we can see the efficiency of Zinc in the reduction of alkynes.
For more reference, please click on:
http://www.citeulike.org/user/hongan1985/article/279061
Wednesday, August 6, 2008
Zinc reduction of alkynes chymistry
Labels:
chemistry info
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment